Read FIN’s latest updates, upcoming events and all news relating to Fabry Disease here
April is Fabry Awareness Month!
April is Fabry Awareness Month! Join FIN in celebrating the #FabryHeroes!
FIN FABRY AWARD 2025
FIN Award 2024 Applications now open!
Live Webinar Arrhythmia in Fabry & what can we do about it?
Join us for the live webinar with Dr Ashwin Roy 'Arrhythmia in Fabry & what can we do about it' on November 13th at 6pm CET
Save the date – FIN Fabry Expert Meeting April 25-26, 2025
Save the date - FIN Fabry Expert Meeting April 25-26, 2025
Save the date – FIN Fabry Expert Meeting April 24-25, 2026
Save the date - FIN Fabry Expert Meeting April 25-26, 2025
April is Fabry Awareness Month!
April is Fabry Awareness Month! Join FIN in celebrating the #FabryHeroes!
Webinar Fabry Highlights WorldSymposium 2024
Join us for the Fabry Highlights Worldsymposium 2024 Webinar with Dr Hopkin Feb 14th , 2023 at 6pm-7pm CET
Message from Sangamo
Message from Sangamo to the Fabry community
FIN AWARD 2023
FIN Award 2024 Applications now open!
FIN Fabry Expert Meeting 2024
Registration now open - FIN Fabry Expert Meeting 2024, April 19-20 in Prague, Czech Republic
FIN Fabry Young Adults Webinar on November 18th at 1pm CET
Join us for the next FIN Fabry Young Adults Webinar on November 18th at 1pm CET
Webinar on Gene Therapy with Dr Hughes on November 13th at 6.30pm CET
Join us for a webinar on Gene Therapy with Dr Derralynn Hughes on November 13th at 6.30pm CET
Save the date – FIN Fabry Expert Meeting April 19-21, 2024
Save the date - FIN Fabry Expert Meeting April 19-21, 2024
Two open positions at the FIN Board
Two open positions at the FIN Board. Apply now!
Meeting Report FIN Fabry Expert Meeting 2023
Meeting report FIN Fabry Expert Meeting 2023 now available
CARAT study now recruiting
PERIDOT study now recruiting
PERIDOT study now recruiting
PERIDOT study now recruiting
New FIN President
Lut de Baere resigned as FIN President, Mary Pavlou elected as successor!
News from Chiesi
Chiesi Global Rare Diseases and Protalix BioTherapeutics Announce European Commission Authorization of PRX-102 (pegunigalsidase alfa) for the Treatment of Fabry Disease
News from Sangamo
The STAAR Study is Recruiting Participants Now
News from Sanofi
Sanofi dedicated their Fabry Awareness Month Campaign to highlighting the psychological impact of Fabry.
Fabry Outcome Survey- Final Report 2022
I am pleased to [...]
News from Freeline
Freeline has made the difficult decision to not progress our Fabry disease program further
April is Fabry Awareness Month!
April is Fabry Awareness Month! Join FIN in celebrating the #FabryHeroes!
News from Sanofi
Orphanet Journal of Rare Diseases recently published an in-depth history of the Sanofi Rare Disease Registries.
Sad news from Fabry Korea
It is with great sadness that we learned of the passing of BongKi Lim, Fabry Korea's chairman for the past 22 years.
Evaluating the direct and indirect costs of Fabry disease
Evaluating the direct and indirect costs of Fabry disease
News from Sangamo
Chiesi Global Rare Diseases and Protalix BioTherapeutics Receive Positive CHMP Opinion for Pegunigalsidase Alfa for Treatment of Fabry Disease.
News from Chiesi
Chiesi Global Rare Diseases and Protalix BioTherapeutics Receive Positive CHMP Opinion for Pegunigalsidase Alfa for Treatment of Fabry Disease.
Webinar Fabry Highlights of the WorldSymposium 2023
Join us for the Fabry Highlights from the Worldsymposium 2023 webinar with Dr Hopkin April 5th, 2023 at 6.30pm-7.30pm CET
Fabry Women’s Day is coming up!
April 1st is International Fabry Women’s Day! Join us in celebrating this special day.
A solid partnership with strong ideas
Putting people with Fabry first: a talent that Amicus [...]
Putting Fabry on Australia’s agenda
Fabry disease is rather easy to test for and [...]
Rare Diseases International Annual Membership Meeting
Rare Diseases International Annual Membership Meeting
An update from Fabry China
An update from Fabry China
Fabry community leadership in an evolving landscape
Fabry community leadership in an evolving landscape
International Fabry Congress MPS Lysosomales Association Spain
International Fabry Congress MPS Lysosomales Association Spain
Help us to train Face2Gene to diagnose Fabry patients earlier!
Help us to train Face2Gene to diagnose Fabry patients earlier!
News from Avrobio
Letter to the Fabry Community from Avrobio
News from Sangamo Therapeutics
Sangamo Therapeutics Announces Updated Preliminary Phase 1/2 Data Showing Tolerability and Sustained Elevated α-Gal A Enzyme Activity in Patients With Fabry Disease
Webinar Fabry Highlights of the WorldSymposium 2023
Join us for the Fabry Highlights from the Worldsymposium Webinar with Dr Hopkin April 5th, 2023 at 6.30pm-7.30pm CET
Fabry Expert Meeting 2022 – Virtual on May 7th
Virtual Fabry Expert Meeting 2022 on May 7th
Addressing the Challenges of Persons Living with a Rare Disease
Addressing the Challenges of Persons Living with a Rare Disease
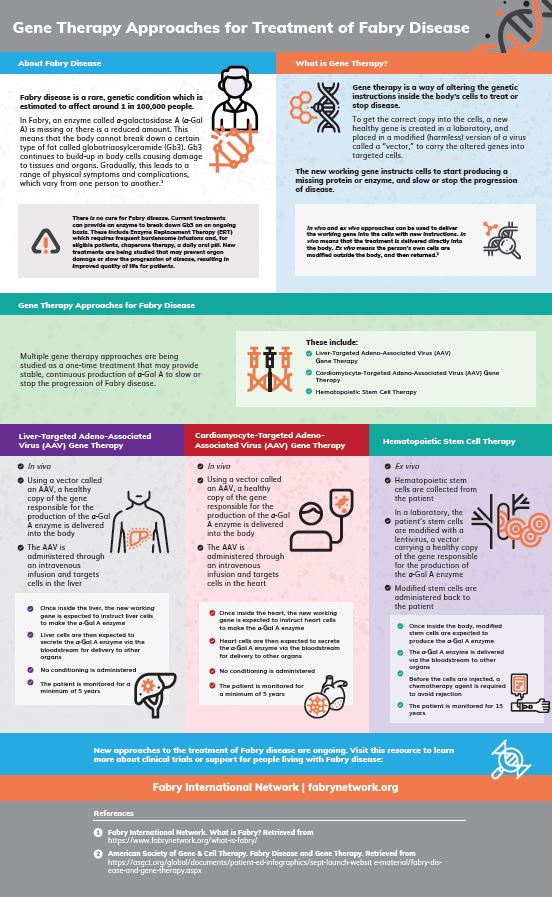
Gene Therapy Approaches for Fabry Diseases
We're are happy to [...]
News from Freeline
Freeline announced new data from its ongoing Phase 1/2 MARVEL-1 dose-finding clinical trial of FLT190 for the treatment of Fabry disease and provided updates on its pipeline programs.
News from Idorsia
Idorsia Ltd (SIX: IDIA) today announced that after the planned interim analysis of the open-label extension (OLE) of the Phase 3 MODIFY study with lucerastat for the treatment of adult patients with Fabry disease, the study will continue.
The African Summit on Rare Diseases
The African Summit on Rare Diseases 2021
FIN AWARD 2022
FIN AWARD 2022 Applications now open!
News from Sangamo Therapeutics
Sangamo Therapeutics Announces Preliminary Phase 1/2 Data Showing Tolerability and Sustained Elevated α-Gal A Enzyme Activity in Patients With Fabry Disease
News from Takeda: Update to FOS Registry
News from Takeda: Update to FOS Registry
Webinar: Fabry & the Brain
Fabry Findings Webinar: Fabry & the Brain now available on our YouTube Channel
Fabry Outcome Survey- Annual Report 2020
Throughout 2020, the COVID-19 [...]
A new tool to capture symptoms for Fabry Disease?
In December 2020 and [...]
Fabry Findings Webinar
Fabry Findings Webinar - October 27th, 2021 - 5.30pm - 6.30pm CEST We are happy to invite you to the first Fabry Findings Webinar with Dr Simon Korver who will present the findings from our first issue.!
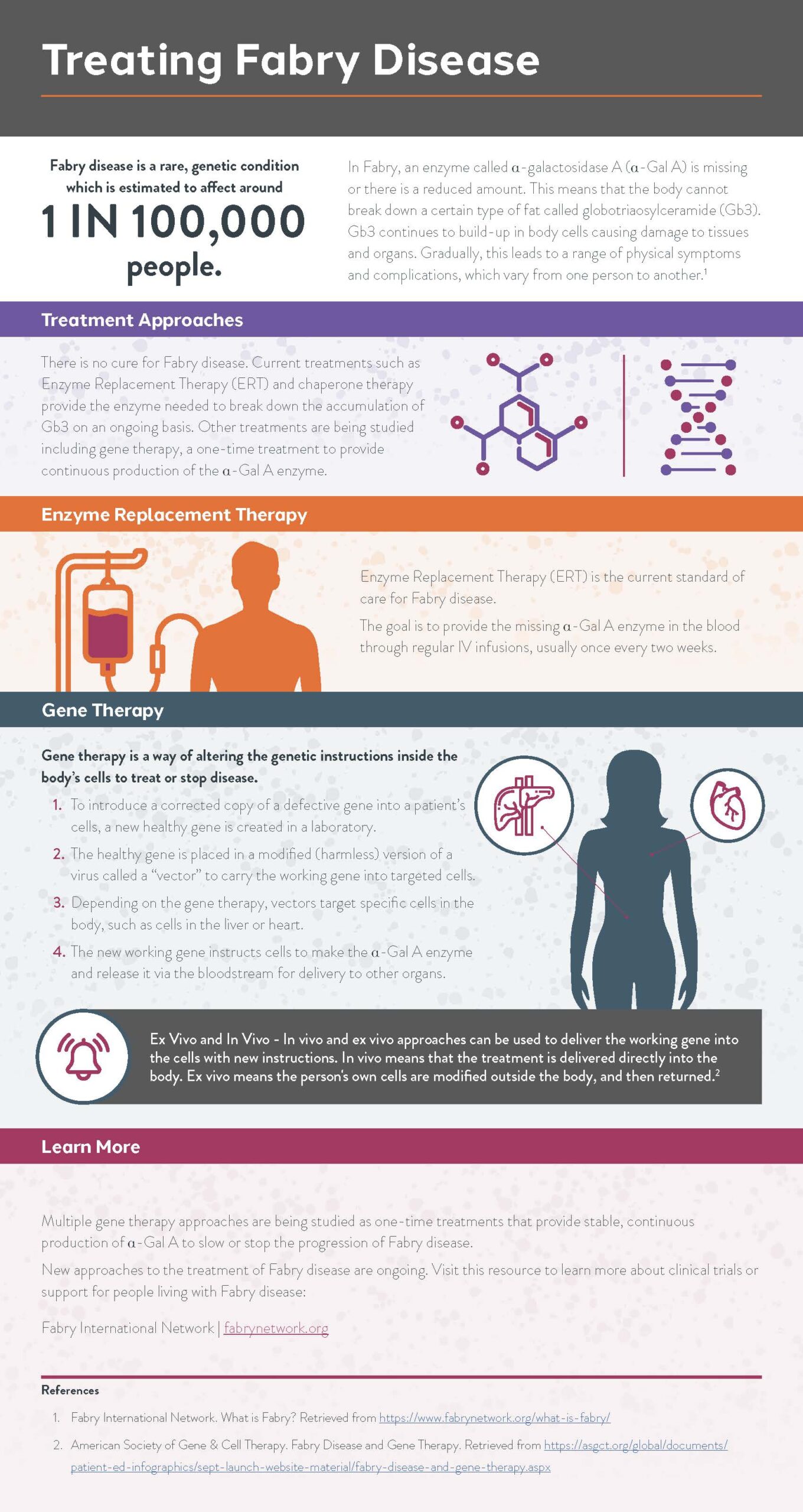
What is Gene Therapy?
Gene therapy is a [...]
Understanding the importance of shared health care decisions
Health care decisions are never easy to [...]
Amicus Therapeutics Announces European Commission Approval of Galafold® (migalastat) for Adolescents with Fabry Disease
Amicus Therapeutics Announces European Commission Approval of Galafold® (migalastat) for Adolescents with Fabry Disease
The study, “Frequency of Fabry disease in a juvenile idiopathic arthritis cohort,” was published in the journal Pediatric Rheumatology
The study, “Frequency of Fabry disease in a juvenile idiopathic arthritis cohort,” was published in the journal Pediatric Rheumatology.
AVROBIO AVR-RD-01 Gene Therapy Trial Now Open to Female Patients
AVROBIO Provides Regulatory Update on Investigational AVR-RD-01 for Fabry Disease
Nordic Rare Disease Summit
The Nordic Rare Disease Summit, organized as a virtual meeting on 12th and 13th of April 2021, gathered a wide range of rare disease experts, decision- and policymakers as well as representatives from NGO’s, patient organizations, academia and industry from across the Nordic countries
AVROBIO Provides Regulatory Update on Investigational AVR-RD-01 for Fabry Disease
AVROBIO Provides Regulatory Update on Investigational AVR-RD-01 for Fabry Disease
MPS Spain – Fabry Congress 2021 on June 17-18, 18:00h
The MPS - Mucopolisacaridosis y Síndromes Relacionados is organising their virtual international Fabry Congress 2021 on June 17-18, 18:00h. (CET)! They have an exciting program lined up with worldwide Fabry Experts in English and Spanish!
Open letter to Fabry Centres
To highlight the need for continuous care and monitoring for Fabry disease patients and remind everyone of action needed for newly diagnosed patients, those in the midst of the diagnostic process or those experiencing symptoms for the first time and needing assessment We want to emphasise the need for ongoing clear and consistent communication from the centres about patients’ care and having regular appointments (in-person or telehealth)
#Break A Sweat For Fabry
This year we challenge you to "Break A Sweat For Fabry" and post your picture on social media with the hashtag #BreakASweatForFabry. By breaking a sweat for people who have difficulties sweating or can't sweat at all, you can show your support and help raise awareness.
Welcome to the GRIT Study
Getting global Rare disease Insights through Technology Join the first Canadian app-based clinical trial for patient with metabolic disorders
Zamplo
Zamplo and Fabry International Network are teaming up to introduce the Zamplo app to individuals living with Fabry disease and their caregivers.
Fabry Women’s Day is coming up!
April 3rd is International Fabry Women’s Day! Join us in celebrating this special day.
FIN Expert Meeting 2021
Registration now open We are very pleased to invite you to our first online FIN Expert Meeting on April 24th, 2021!
Treatment needs and expectations for Fabry disease in France
development of a new Patient Needs Questionnaire In France, two associations actively represent Fabry patients, participate in and promote medical research: Association des Patients de la Maladie de Fabry (APMF, apmf-fabry.org) and Vaincre les Maladies Lysosomales (VML, www.vml-asso.org).
Learning from the Pandemic to Improve Care for Vulnerable Communities
The Perspectives and Recommendations from the Rare Disease Community' Raquel Castro, Erwan Berjonneau and Sandra Courbier from Eurordis have published an editorial piece on the International Journal of Integrated Care entitled: